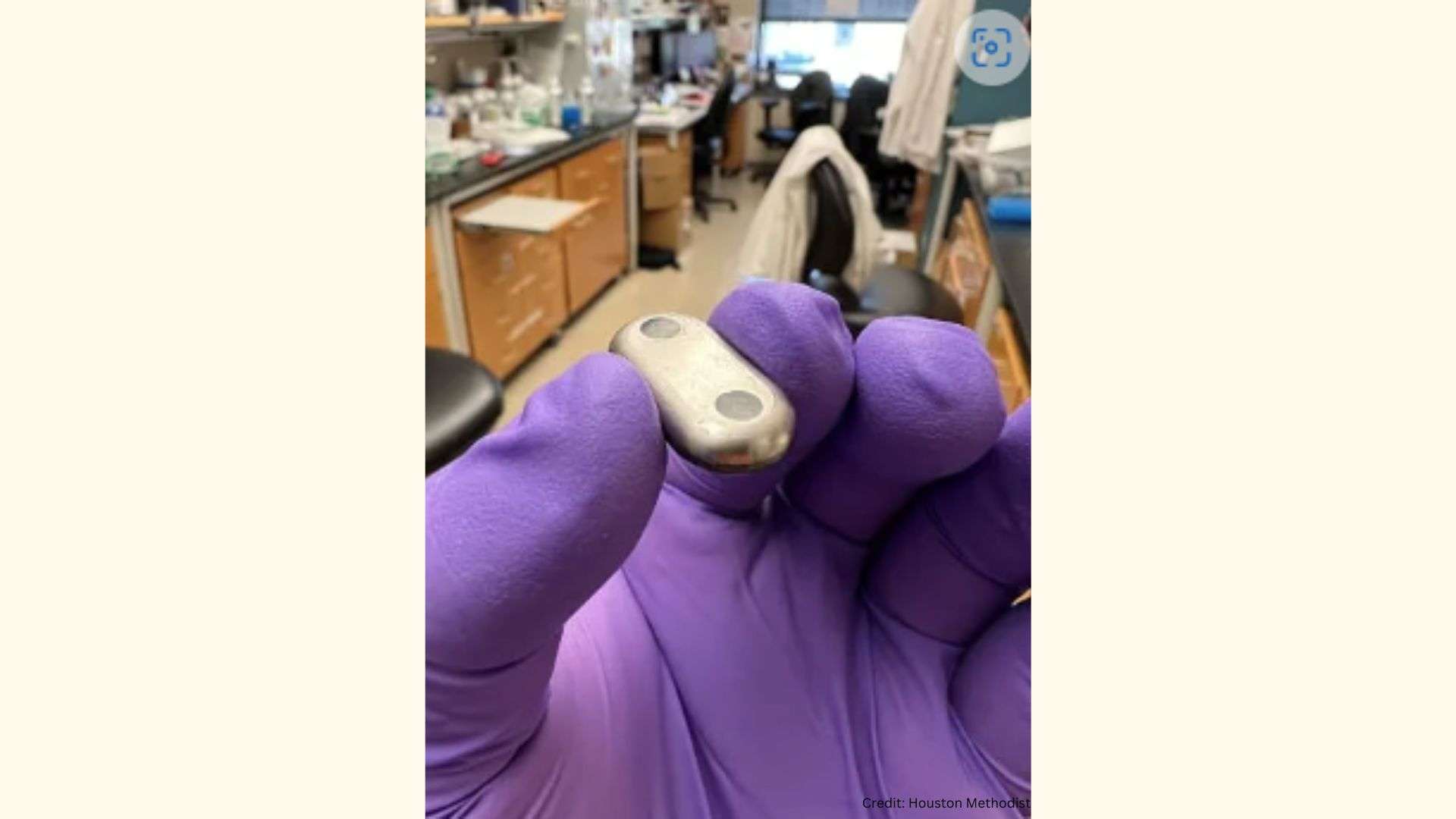
HOUSTON — A team from Houston Methodist Research Institute recently showed that a nanofluidic implant delivered an HIV drug that achieved more potency than other forms of drug administration (oral) and other HIV drugs.
The results, published in The Journal of Controlled Release, may lead to a long-acting, refillable implant that delivers an HIV antiviral medication, Islatravir, into humans living with human immunodeficiency virus (HIV), a chronic yet manageable disease.
Currently, Islatravir is an investigational drug administered once daily or weekly in a combination regimen. This research, led by Alessandro Grattoni, Ph.D., chair of the Department of Nanomedicine at Houston Methodist Research Institute, focused on the potency, efficacy and tolerability of the drug when subcutaneously administered at a low dose in a continuous manner.
“The potency of continuous, subdermal elution of Islatravir was 5-fold higher than cabotegravir, an intramuscular injectable antiviral for HIV prevention and treatment,” said Grattoni, the study’s corresponding author.
Treatment nonadherence remains a prevalent challenge for many who take HIV medications, which can eventually lead to patients’ inability to tolerate antiretrovirals, an HIV infection prevention protocol that has been around for more than a decade.
However, as with all treatments, combination drugs are needed for optimal treatment efficacy.
The Houston Methodist researchers are also studying this same implant for delivering HIV prevention medications.
The nanofluidic device is intended for long-term controlled and sustained release, avoiding repeated systemic treatment that often leads to adverse side effects. Additional lab research is underway to determine the effectiveness and safety of this delivery technology, but researchers would like to see this become a viable option for HIV patients in the next few years.
By: Houston Methodist